Renal Hypoxia: Why kidney is prone to hypoxia and ischemic injury?
Renal hypoxia can lead to acute kidney injury and chronic kidney disease.
The kidneys weigh less than 0.5% of body weight and receive 20%–25% of our cardiac output at rest. The kidneys are the most highly perfused organs in the body if we calculate blood per gram of the tissue.
Despite having an ample supply of blood, the kidney especially, the renal medulla is highly vulnerable to hypoxia and subsequent ischemia. Hypoxia and ischemia are the significant pathophysiological features of acute kidney injury and chronic kidney disease.
The S3 segment (straight segment) of the proximal tubule and the medullary thick ascending limb of the loop of Henle (mTAL) are most prone to ischemic injury. Both these tubular areas exist in relatively lower oxygen conditions. It is a trade-off to be able to get a high osmotic gradient and concentrated urine.
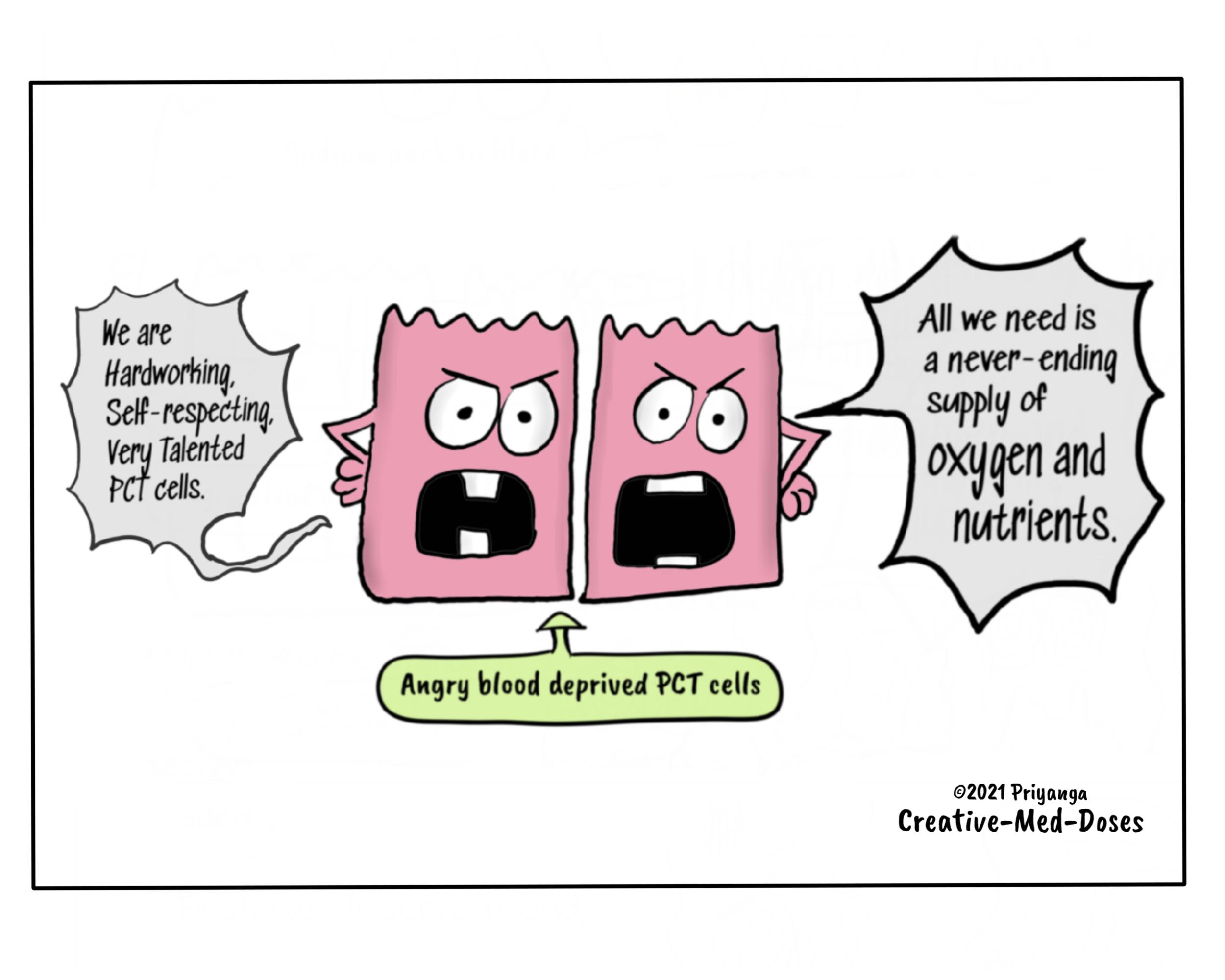
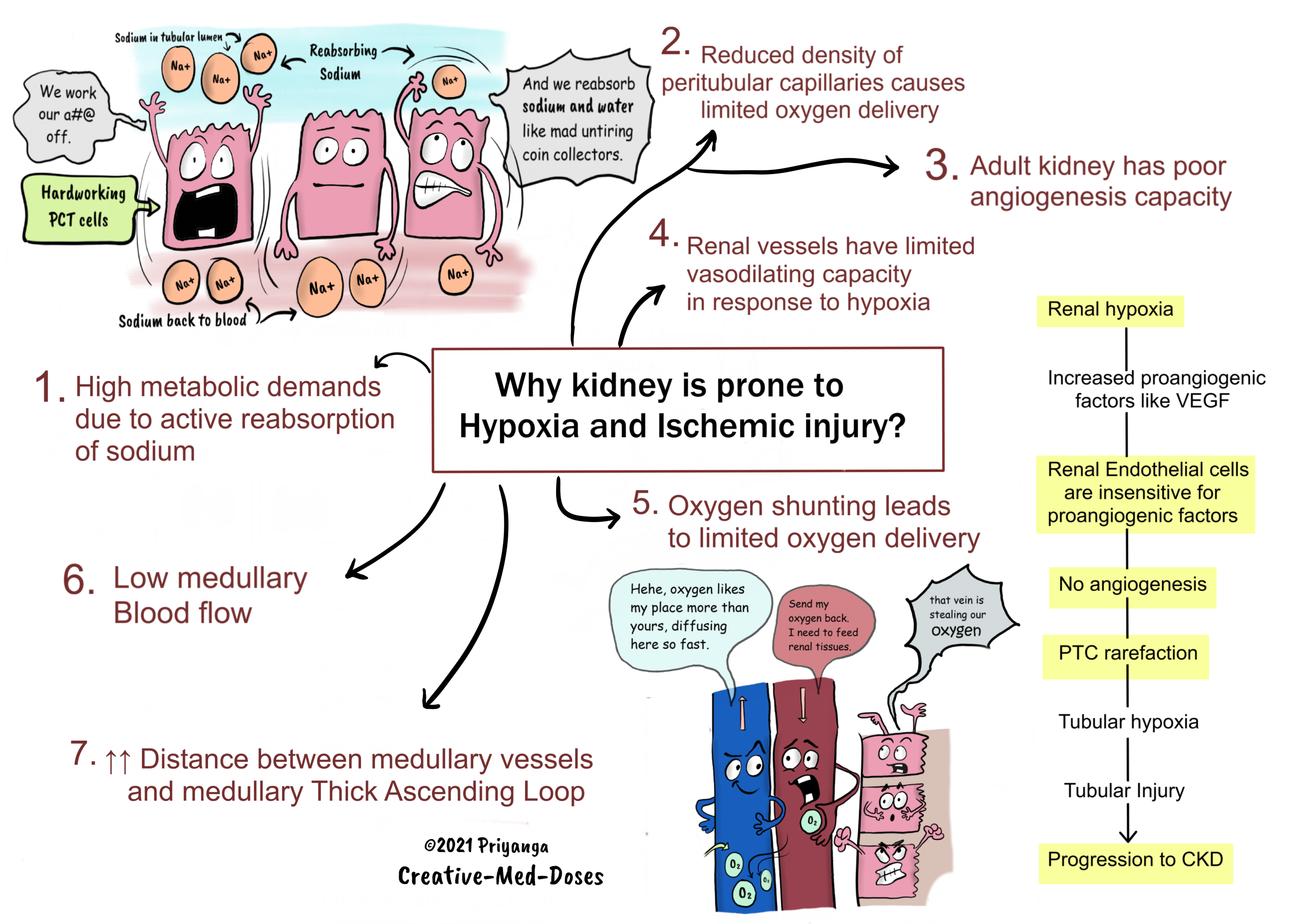
1.Renal tubules have high metabolic demands due to active reabsorption of sodium -Metabolic demands of renal tubules keep them hungry for oxygen, especially proximal convoluted tubules of the renal medulla are always on the verge of getting hypoxia.
The proximal convoluted tubules reabsorb most of sodium and water from tubular filtrate. Sodium reabsorption is an active process that requires energy in the form of ATP. The production of ATP is dependent upon oxygen supply. PCT cells are always in demand of oxygen, and anything which compromises the oxygen delivery to these cells causes PCT cell injury and death.
...
...
2.Reduced density of peritubular capillaries causes limited oxygen delivery-
Chronic kidney disease cases have capillary rarefaction. It impairs oxygen delivery and causes further tubular damage. Such kidneys are in a vicious cycle of tubular injury, inflammation, interstitial fibrosis, and capillary loss.
Peritubular capillary (PTC) rarefaction is one of the hallmarks of CKD, and the degree of PTC loss may predict the renal outcome.
PTC endothelial cells (ECs) undergo apoptosis during CKD, leading to capillary loss, tissue hypoxia, and oxidative stress.
In a normal kidney, there is a perfect balance of proangiogenic factors and anti-angiogenic factors. It maintains renal angiogenesis to the optimum level. In cases with CKD, tubular injury leads to endothelial damage and apoptosis, Which causes reduced proangiogenic factors and increased anti-angiogenic factors. It limits the kidney’s ability to recruit more capillaries in hours of need.
The endothelial injury and interstitial edema caused by tubular injury lead to vascular collapse. Reduced peritubular capillary numbers render tubules sensitive for oxygen deprivation and subsequent hypoxia. Capillary rarefaction is one of the reasons why CKD cases have an increased risk of developing acute kidney injury.
Note: Mitigating PTC rarefaction might become the practical therapeutic target to halt the progression of CKD.
3.Adult kidney has poor angiogenesis capacity
Renal endothelial cells are relatively insensitive to proangiogenic factors like VEGF (vascular endothelial growth factor.
Endothelial cells of the kidney have a poor proliferative capacity which leads to peritubular rarefaction.
It increases the risk of progression of acute kidney injury to chronic kidney disease.
4.Renal vessels have limited vasodilating capacity in response to hypoxia
Most organs under hypoxic conditions increase local blood flow by vasodilation (Functional/Reactive Hyperemia). But the renal vessels do not dilate in response to acute hypoxia. Since the kidney is the body’s critmeter and erythropoietin producer, the kidney tends to preserve these functions at the cost of protecting itself from hypoxia.
If the kidney vasodilates in response to every acute hypoxia episode, it will immediately change the Glomerular filtration rate. And extracellular fluid volume will be affected, which seems to affect every organ of the body afterward. In short, evolution has put more value on the regulation of ECF volume than it has on saving kidneys from hypoxia.
5.Oxygen shunting leads to limited oxygen delivery -
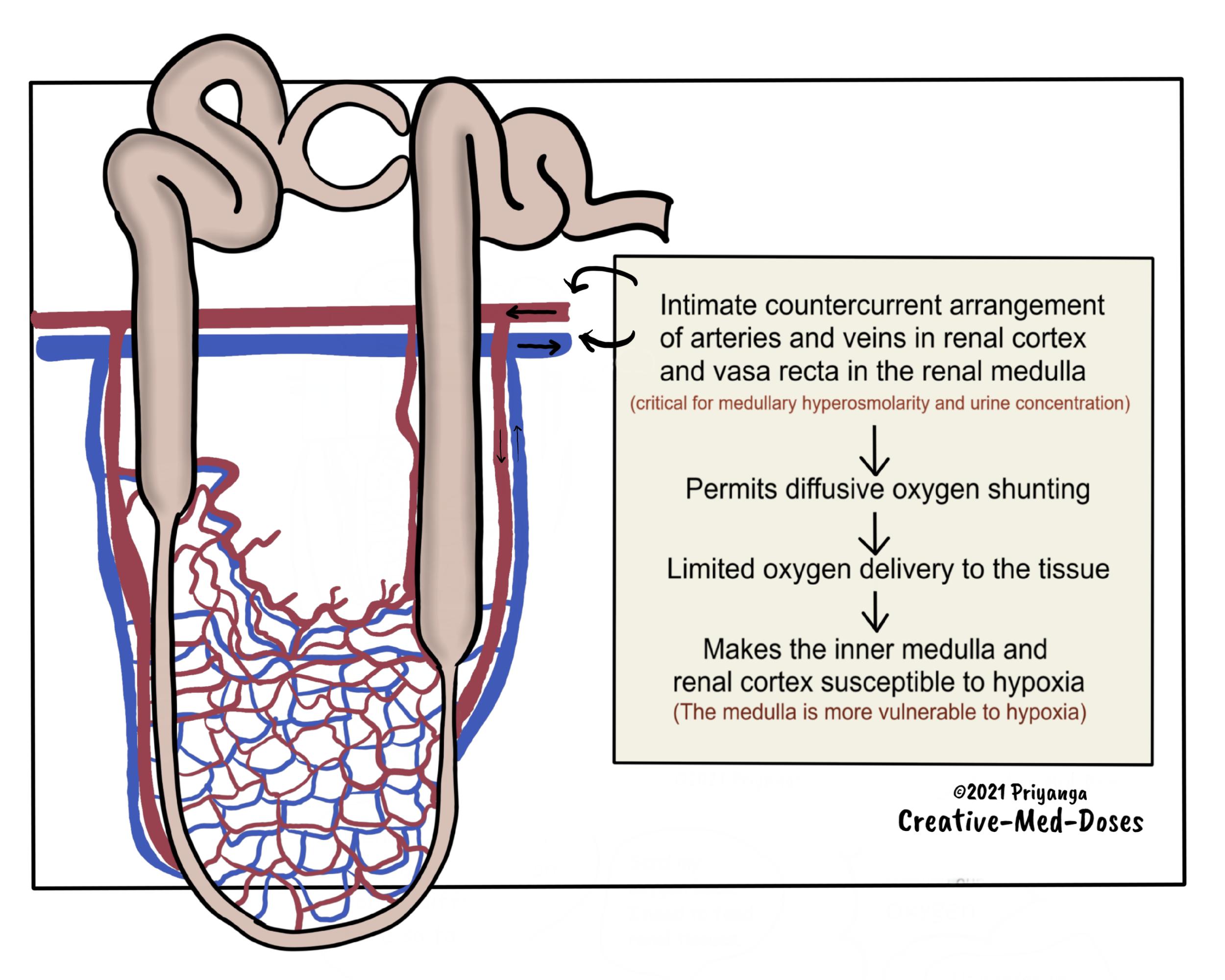
The branches of the renal arteries and veins run parallel and in close contact with each other in the renal cortex and the medulla. The blood flows in countercurrent fashion in these intimately located vessels.
This parallel arrangement facilitates oxygen to diffuse from the arterial system into the venous system before it has entered the tubular capillary bed. It is called diffusive oxygen shunting.
This oxygen shunting deprives renal tubules of oxygen and makes them vulnerable to hypoxia. The low oxygen supply impairs energy generation (ATP formation) in tubules.
Most of the tubular segments (proximal tubule and medullary thick ascending loop) have a limited capacity for anaerobic energy generation. And are dependent on oxygen for aerobic energy generation to maintain active tubular reabsorption of solutes, especially sodium.
The limited tissue oxygen supply and high oxygen demand increase the susceptibility of the kidney to hypoxia, ischemia, and acute kidney injury.
...
...
6.Low medullary flow to facilitate urine concentration makes the medulla vulnerable to hypoxia-
The generation of a gradient of increasing osmolality from the outer medulla to the tip of the papilla is a critical component of the urinary concentrating process.
The countercurrent arrangement of descending and ascending vasa recta creates an osmotic gradient.
The renal medulla's low blood flow is an absolute requirement for the maintenance of this solute gradient. Medullary blood flow is <1% of total renal blood flow.
If the medullary blood flow increases, the efficiency of a countercurrent system falls. Because of the reduction in transit time, the solute cannot concentrate in the medulla to create an osmotic gradient.
Low blood flow causes slow oxygen delivery, which can cause a discrepancy in medullary oxygen demand and supply. That makes the renal medulla vulnerable to hypoxia.
7.Distance between medullary vessels and medullary Thick Ascending Loop (mTAL)
Tubular-Vascular relationship in the medulla is critical for countercurrent exchange and maintenance of osmotic gradient.
The medullary thick ascending loop of Henle (mTAL) is a little distant from descending vasa recta.
According to the hypothesis, this topography has an adaptive benefit of an increased lateral osmotic gradient. It promotes sodium and water reabsorption in the thick ascending loop of Henle.
Having blood vessels a little far limits blood supply and oxygen delivery to mTAL. The mTAL has high metabolic demands because it actively reabsorbs sodium.
The high demand and sparse oxygen supply render mTAL vulnerable to hypoxia, ischemia, and acute kidney injury.
...
...
Revision for today Capillary Leak Syndrome (CLS): Quick Review - Creative Med Doses
Buy fun review books here (these are kindle eBook’s you can download kindle on any digital device and login with Amazon accounts to read them). Have fun and please leave review.