Inflammatory Bowel Disease (IBD): Pathogenesis
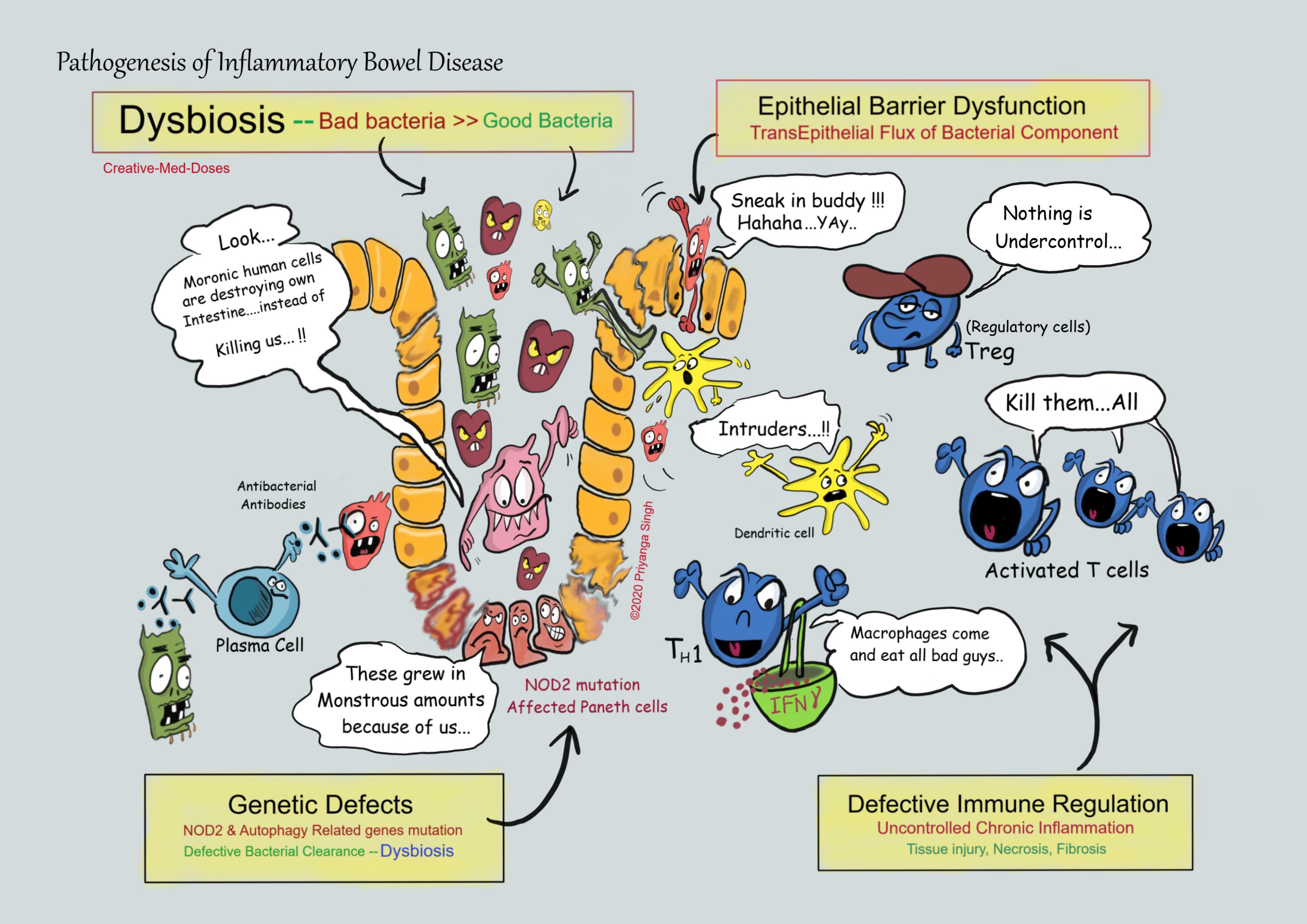
Inflammatory bowel disease is a chronic relapsing inflammatory disorder of intestine. It includes two major forms, Crohn’s disease and ulcerative colitis. Although the exact etiology of IBD is unknown, it involves a complex interaction between the genetic, environmental or microbial factors and the immune responses.
Following are major factors associated with Inflammatory Bowel Disease-
Genetic predisposition –
There are multiple genes involved but following are commonly associated with IBD-
NOD2 Polymorphism- NOD2 (nucleotide-binding oligomerization domain) plays a key role in the regulation of microbiota in the small intestine. NOD2 is highly expressed in ileal Paneth cells that provide critical mechanism for the regulation of ileal microbiota through the secretion of anti-bacterial compounds.
NOD2 senses microbiota-derived products and activates NF-κB which is critical for the bacterial killing activity of Paneth cells via the secretion of anti-bacterial compounds.
Loss of function NOD2 mutants disturb many characteristics of gut immune homeostasis, including reduced bacterial product sensing, and impaired antimicrobial responses in Paneth cells, leading to defective bacterial clearance. It creates dysbiosis in microbiota of intestine which is, characterized by increased load of bacteria and activates immune system for continuous attack.
Autophagy Related Genes - Autophagy is involved in intracellular homeostasis, contributing to the degradation and recycling of cytosolic contents and organelles, as well as to the resistance against infection and removal of intracellular microbes. There are two autophagy-related genes named ATG16L1 and IRGM are defective in Epithelial cells and dendritic cells leading to accumulation of microbial contents further aggravating the inflammation.
Dysbiosis-
The Changes in endogenous commensal microbiota within the intestines plays a central role in the pathogenesis of IBD, also called state of “Dysbiosis”.
The quantity of microbial organisms in human intestine is enormous and every individual has unique set of microorganisms. The composition of these commensals is strikingly important for gut health. When amounts of beneficial bacteria is less in comparison to bad bacteria there is innate and adaptive immune response, in case of IBD it becomes out of proportion because of genetic predisposition, dietary and smoking habits as well as stressful lifestyle. These bacteria and its components cause disruption in epithelial barrier of intestine, leading to transepithelial Flux of luminal bacterial components and stimulating inflammatory cells.
Epithelial Barrier Dysfunction -
Defects in intestinal epithelial tight junction barrier function occur in patients with IBD, which leads to accumulation of bacterial components inside intestinal wall and stimulate dendritic cells and T cell reactions.
Defective Immune Regulation -
Dysfunctions of innate and adaptive immune pathways contribute to the aberrant intestinal inflammatory response in patients with IBD. Defects in regulatory T cells, especially the IL-10 producing T cells are main initiators. Mutations in the IL-10 receptor are associated with severe, early-onset colitis. Excessive production of TH1 and TH17 cell and its activity inducing cytokines causes lymphoid aggregation, more T cell activity. TNF and IFN gamma are secreted which further causes fibrosis, necrosis and inflammation.
...
...
Thus, some combination of excessive immune activation by intestinal microbes and defective immune regulation likely is responsible for the chronic inflammation in IBD.
Stay tuned for more fun maps. Subscribe and share ❤ if you like them.
Further reading https://academic.oup.com/ibdjournal/article/12/suppl_1/S3/4676576
Revision for today https://creativemeddoses.com/topics-list/marfan-syndrome-quick-review/